WAYS OF INVESTIGATING THE BRAIN
SPECIFICATION:
There are several ways of studying the brain, including scanning techniques, including functional magnetic resonance imaging (fMRI), electroencephalograms (EEGs), event-related potentials (ERPs), and post-mortem examinations.
SCAN A BRAIN
https://fi.edu/en/neuroscience-and-society/media/interactive/scan-a-brain
INTRODUCTION TO BRAIN RESEARCH METHODS
We recognise the brain as central to understanding behaviour today, but this clarity wasn’t always available. Without advanced tools like fMRIs or microscopes, early scholars relied on philosophy and reasoning to explain human nature. For instance, the Ancient Egyptians valued the heart more than the brain, and mental illness was often attributed to supernatural causes like demonic possession. This changed with technological advancements, shifting from speculative philosophy to empirical methods. Tools like microscopes, electrical stimulation, and neuroimaging have revolutionised our understanding of brain function, moving science forward.
SEEING IS BELIEVING
As our methods evolved, so did our knowledge. John Locke, a key empiricist, believed that proper understanding requires sensory data and specialised tools to observe phenomena the human senses couldn’t directly perceive. The journey from pure reasoning to direct observation, and later to advanced research tools like MRIs and EEGs, reflects the transformation of knowledge—turning mystical ideas into scientifically tested theories.
Now, scientists not only "see" the brain’s structure but also its dynamic functions and their influence on cognition and behaviour.
THE HISTORY OF BRAIN TECHNIQUES
The study of the brain and its connection to behaviour has evolved significantly throughout human history. In ancient times, explanations for unusual behaviour and mental illness were often rooted in superstition. Many cultures believed that mental illness or abnormal behaviour was caused by evil spirits, witchcraft, or demonic possession. In such societies, spiritual rituals, exorcisms, and sometimes tragic consequences such as executions were the primary responses to individuals exhibiting these behaviours.
As civilisations developed, so too did their understanding of the brain. The Ancient Egyptians, while advanced in many fields, held the belief that the heart, rather than the brain, was the seat of thought, emotion, and memory. Meanwhile, the Ancient Greeks took a more rational approach. Influential figures such as Hippocrates began to challenge supernatural ideas, suggesting that mental illness was not due to spirits but instead to physical imbalances within the body. However, it wasn’t until Galen, the Roman physician, that more tangible links between brain function and behaviour began to take shape.
Moving forward into the Renaissance and Enlightenment periods, a gradual shift from spiritual explanations to more empirical methods occurred. The use of dissection and anatomical study by figures such as Vesalius laid the groundwork for future scientific exploration of the brain. By the 19th century, phrenology attempted to link the shape of the skull with personality traits, but this was eventually discredited as pseudoscience.
With the arrival of the modern scientific era, brain research entered a new phase of precision and evidence-based study. Techniques such as ablation and lesion studies began to identify specific brain areas responsible for different functions. Paul Broca, using post-mortem studies, linked damage to certain brain areas with language deficits, contributing to early localisation theories. The development of electrical stimulation in the 19th century demonstrated the brain’s electrical nature, further advancing the understanding of neural activity.
By the 20th century, techniques such as EEG allowed researchers to record brain wave activity and delve deeper into states of consciousness, including the discovery of REM sleep. With the advent of neuroimaging techniques like CT and MRI in the 1970s, scientists were able to non-invasively examine the brain in remarkable detail. Later developments such as fMRI and PET scans revealed not just the brain’s structure but its activity in real time, revolutionising cognitive neuroscience.
The 21st century brought cutting-edge methods like optogenetics, which allows researchers to control the activity of neurons with light, and transcranial magnetic stimulation (TMS), a non-invasive technique for stimulating or inhibiting brain regions, offering both research and therapeutic potential.
INVASIVE & NON INVASIVE WAYS OF INVESTIGATING THE BRAIN
Researching the brain can be broadly divided into two main types: invasive and non-invasive methods.
INVASIVE METHODS: involve physically interacting with the brain, often through surgery, the application of chemicals, or electrical stimulation. These techniques may require removing, damaging, or manipulating brain tissue to observe the effects on behaviour, brain function, or neurological processes. Though highly informative, these approaches are usually limited to animal studies or, historically, in extreme cases of human treatment.
NON INVASIVE METHODS: on the other hand, allow researchers to study the brain without physically altering or damaging it. These techniques, such as MRI or EEG, use external equipment to monitor brain activity or visualise brain structures. Noninvasive methods are typically safer and more widely used in human research, and they provide valuable insights into brain function and behaviour without the need for surgical intervention.
INVASIVE METHODS OF INVESTIGATING THE BRAIN
LESIONS AND ABLATIONS
TIME: 19th century onwards
DESCRIPTION: Lesions and ablations are methods used to intentionally damage or remove parts of the brain to study their functions. In ablations, tissue is either surgically removed (aspiration) or destroyed without removal (which is common, despite the term “ablation” originally meaning “carried away” in Latin). Lesions involve damage to specific areas through various techniques:
Aspiration (ablation): The surface of the cortex is cut or sliced away using suction.
Radiofrequency lesion: An electrode is inserted into the target location and heated to destroy nearby cells.
Neurotoxic lesion: A neurotoxin like biogenic or kainic acid is injected to destroy neurons without damaging nearby fibres.
Surgical cutting: Brain tracts can be surgically cut to sever connections, disrupting communication between regions.
Reversible lesions: This is achieved by cooling or injecting chemicals (like lidocaine) to temporarily disable a brain area, allowing researchers to reverse the lesion and observe changes before and after.
RESEARCH FROM THIS TECHNIQUE: Pierre Flourens pioneered the ablation technique, revealing that the cerebellum is critical for motor coordination and that the brainstem controls essential life functions like breathing. In modern studies, lesions have helped researchers map the motor cortex and better understand brain circuits involved in movement and sensation. Neurotoxic lesions have provided important insights into diseases like Parkinson's disease by selectively destroying dopamine neurons while cutting brain tracts has been instrumental in commissurotomies (cutting the corpus callosum) to treat epilepsy.
ADVANTAGES:
Precise targeting: Stereotaxic equipment allows for exact cuts or lesions in targeted brain regions, enabling specific investigations into brain function.
Flexibility of methods: Different types of lesions (radiofrequency, neurotoxic, aspiration) allow researchers to target cell bodies, fibres, or entire regions.
Reversible lesions: Provide a dynamic way to observe changes in brain function without causing permanent damage.
DISADVANTAGES:
Invasiveness: Lesions and ablations cause irreversible damage to brain tissue, raising ethical concerns, especially in animal research.
Interconnected brain systems: Damaging one area may disrupt connections between regions, making isolating the lesion's effects difficult.
Uncertainty in behaviour: It can be hard to confirm whether the observed behavioural changes are solely due to the lesion or are secondary effects.
Compensatory mechanisms: After damage, other brain areas may compensate, masking the full impact of the lesion.
ELECTRICAL STIMULATION AND NEURAL INHIBITION IN THE BRAIN
TIME: Mid-19th century onwards
DESCRIPTION: This technique involves applying an electrical or chemical signal to specific regions of a neural circuit to either activate or inhibit brain activity. In animal studies, electrodes are inserted into the brain, and a weak electric current is applied to mimic nerve impulses. This causes the brain to respond as if receiving real sensory input. In humans, electrical stimulation is often used during neurosurgery to map brain functions, ensuring that critical areas such as language involvement are not damaged. Inhibition techniques can block activity in targeted areas, helping researchers assess the functional significance of these regions.
RESEARCH FROM THIS TECHNIQUE: Electrical stimulation has been critical in mapping the motor cortex, where stimulation of specific regions leads to movements in corresponding body parts. This has contributed to our understanding of the localisation of motor control. However, the technique is less effective for studying regions like the somatosensory cortex, as animals cannot explicitly communicate internal sensory experiences.
ADVANTAGES:
Provides real-time information on brain functions and neural pathways.
It allows researchers to stimulate precise brain areas, helping to localize functions such as movement or sensory processing.
It can be used in neurosurgical settings to minimise damage to critical areas during operations.
DISADVANTAGES:
Invasive: Involves inserting electrodes into the brain, which can lead to discomfort or side effects, especially in animal studies.
Crude: Controlling the extent of stimulation can be difficult, making it difficult to isolate the function of a specific region.
Limited to Observable Behaviours: This technique is highly useful for studying observable behaviours (e.g., motor movements), but less effective for understanding internal experiences or sensory processes since animals cannot verbally report sensations.
Ethical Concerns: In animal studies, ethical concerns arise due to the procedure's invasive nature, which can cause discomfort or distress.
CHEMICAL STIMULATION OF THE BRAIN
TIME: Mid-20th century onwards
DESCRIPTION: Chemical stimulation of the brain involves administering small amounts of psychologically active chemicals (e.g., medications or recreational drugs) directly into specific brain regions through thin cannulas. These chemicals selectively influence different mental processes by acting on specific brain systems. Psychologically active medications (such as antipsychotic medications, anesthetics, and antianxiety drugs), as well as recreational substances (like nicotine, LSD, heroin, and cocaine), affect particular brain circuits. This method allows researchers to pinpoint the location and function of brain systems responsible for mediating the effects of these substances. For example, the stimulant amphetamine affects the same brain system where electrical stimulation produces substantial reinforcing and rewarding effects.
RESEARCH FROM THIS TECHNIQUE: Early studies by Olds (1967) demonstrated that amphetamine directly activates the brain’s reward system, highlighting the overlap between the effects of chemical and electrical stimulation on reinforcement and pleasure. These experiments have been critical in identifying the neural pathways responsible for addiction, motivation, and reward processing.
ADVANTAGES:
Precise control over the administration of specific chemicals allows researchers to isolate and study the effects of particular brain systems.
Direct insight into how different psychoactive substances (both therapeutic and recreational) interact with brain systems, shedding light on their effects on behaviour and mental processes.
It helps understand the mechanisms of action for medications used to treat psychiatric disorders like schizophrenia or anxiety.
DISADVANTAGES:
Invasive: Requires the insertion of cannulas into the brain, which can be uncomfortable or harmful, particularly in animal studies.
Ethical concerns regarding the use of psychoactive chemicals in animal models and the potential for long-term effects on brain function.
Limited generalisability: While chemical stimulation can reveal how specific substances act on particular brain systems, the results are limited to the substances used in the experiment and may not generalise across different chemicals or brain areas.
Non-observable effects: Unlike electrical stimulation, where motor or sensory responses can be directly observed, the effects of chemical stimulation may be internal and more challenging to measure, such as changes in mood or thought processes.
POST-MORTEM STUDIES AND COGNITIVE NEUROPSYCHOLOGY
TIME: 19th century onwards
DESCRIPTION: Post-mortem studies examine the brain after death to connect brain damage or abnormalities to cognitive and behavioural deficits observed during life. Cognitive neuropsychology focuses on studying individuals with accidental brain damage (e.g., from strokes, traumatic brain injuries, or degenerative diseases) to understand how such injuries affect cognition. By analysing the specific impairments caused by these naturally occurring lesions, researchers can infer the function of the damaged regions.
RESEARCH FROM THIS TECHNIQUE: One of the most notable examples of post-mortem research is Paul Broca’s patient, Tan, who lost the ability to speak but retained comprehension. Post-mortem examination of his brain revealed damage to the region now known as Broca’s area, responsible for speech production. Similarly, cognitive neuropsychology cases like Phineas Gage, who suffered frontal lobe damage after an accident, provided insights into the role of the frontal lobe in personality and decision-making. HM, another famous patient, had parts of his hippocampus removed to treat epilepsy, leading to severe memory impairments, furthering our understanding of the hippocampus's role in memory.
ADVANTAGES:
POST-MORTEM STUDIES AND COGNITIVE NEUROPSYCHOLOGYNon-invasive: Relies on naturally occurring brain damage or examination after death, avoiding the ethical concerns associated with more invasive techniques.
Ethically sound: Since the brain damage has occurred naturally or post-mortem, there are no ethical concerns about deliberately harming the brain.
Provides valuable insights into the relationship between brain structure and function, especially when cases involve distinct and circumscribed damage.
DISADVANTAGES:
Limited to static data: Post-mortem studies provide anatomical information but cannot offer real-time data on brain activity or functioning.
Uncontrolled damage: Accidental brain damage is often widespread or imprecise, which makes it difficult to isolate the function of specific brain regions.
Historical behaviour ambiguity: When studying cases of brain damage, it is sometimes difficult to determine how an individual behaved before the injury. For example, if a patient displays aggressive behaviour following damage, it is hard to know if this is a new symptom or if the person exhibited similar behaviour before the injury.
NEUROMODULATION TECHNIQUES
DEEP BRAIN STIMULATION (DBS)
DESCRIPTION:
Deep Brain Stimulation (DBS) is an invasive neuromodulation technique where electrodes are surgically implanted deep within the brain to stimulate specific regions. A small pulse generator, implanted under the skin in the chest, delivers electrical impulses to these electrodes, modulating abnormal brain activity. DBS is primarily used for treating movement disorders, such as Parkinson’s disease, dystonia, and essential tremor, but research is expanding its application to psychiatric disorders like depression and OCD. DBS does not destroy brain tissue and the stimulation can be adjusted or reversed as needed.
ADVANTAGES:
Highly effective for certain disorders: DBS has proven to significantly reduce symptoms of Parkinson's disease, essential tremor, and other motor-related conditions, improving quality of life.
Adjustable and flexible: The electrical impulses can be tailored to the patient’s needs, and the device can be turned off or modified without additional surgery.
Long-term solution: Unlike medications that may lose effectiveness over time, DBS can provide sustained symptom relief for years in many patients.
Preserves brain tissue: Unlike ablative surgeries, DBS does not involve removal or destruction of brain tissue, making it a less drastic option.
DISADVANTAGES:
Highly invasive procedure: DBS requires brain surgery, which comes with risks such as infection, brain hemorrhage, and complications related to anesthesia.
Expensive and resource-intensive: The cost of the DBS device, surgery, and long-term follow-up care is high, requiring a specialized medical team.
Side effects: Some patients may experience side effects such as speech problems, mood changes (e.g., depression or mania), and balance issues as a result of stimulation.
Ongoing maintenance: The implanted device requires regular adjustments and battery replacements, which involve further medical procedures.
IS IT USED IN RESEARCH?:
Yes, DBS is extensively used in research to explore brain function and develop new treatments. Research focuses on:
Psychiatric Disorders: DBS is being studied for conditions such as treatment-resistant depression, obsessive-compulsive disorder (OCD), and even addiction.
Cognitive Neuroscience: Researchers are investigating the effects of DBS on memory, learning, and decision-making by stimulating regions like the hippocampus and prefrontal cortex, aiming to better understand cognitive processes.
Brain Circuit Mapping: DBS is a valuable tool for mapping brain circuits, helping to understand how different regions of the brain interact and respond to stimuli.
New Frontiers: Studies are looking into the use of adaptive DBS that adjusts stimulation in real time based on brain activity, creating personalized, dynamic treatments
NEUROANATOMICAL TECHNIQUES
OVERVIEW:
Neuroanatomical techniques are essential for studying the brain's anatomical structure. While gross examination of the brain can show significant features, these methods allow researchers to investigate cellular details and connectivity within the brain. Brain tissue is preserved and stained using histological procedures so that specific brain parts, such as cell bodies and nerve fibres, can be visualised under a microscope.
HISTOLOGICAL PROCEDURES
Preservation:
After death, autolytic enzymes rapidly degrade the brain's soft tissue. To prevent this destruction, the brain is treated with a fixative, such as formalin, which preserves the tissue. Once preserved, the brain is embedded in a paraffin block, which allows it to be sliced into thin sections using a microtome. These thin slices are mounted onto slides for further examination.
Staining:
Gross examination alone does not reveal the intricate details of brain cells or their connections. Histological stains have been developed to selectively colour parts of the brain, enabling researchers to view specific structures such as cell bodies, nerve fibres, and myelin sheaths under a microscope.
TYPES OF STAINS USED IN NEUROANATOMY
Cell-Body Stains (Nissl Stains)
DESCRIPTION: Cell-body stains, such as the Nissl stain, were developed by Franz Nissl, who discovered that certain dyes, like cresyl violet, would selectively reveal the cell bodies of neurons. These stains allow researchers to view the arrangement and density of neurons in different brain regions.
USE: Nissl stains are particularly useful for identifying areas of the brain where neurons are densely packed and for studying the organisation of brain structures at the cellular level.Myelin Stains
DESCRIPTION: Myelin stains are designed to selectively colour the myelin sheath that surrounds nerve fibres, revealing fibre bundles in the brain. Since myelin helps speed up the transmission of electrical impulses along neurons, these stains are valuable for studying white matter tracts and the brain's communication networks.
USE: Myelin stains help to visualise the white matter of the brain, which is made up of nerve fibres (axons) insulated by myelin. This method is especially helpful in understanding the connectivity between different brain regions.
ADVANTAGES AND DISADVANTAGES OF NEUROANATOMICAL TECHNIQUES
ADVANTAGES:
Detailed cellular insights: These techniques allow researchers to observe the brain's microstructure, including neurons, nerve fibres, and their organisation.
Specificity: Staining procedures can selectively highlight different components of the brain, such as cell bodies or myelinated fibres, helping to map out the brain's detailed architecture.
Long-term preservation: Histological techniques preserve brain tissue for long-term study, enabling comparisons across different brain areas and between individuals or species.
DISADVANTAGES:
Post-mortem analysis: Since these techniques require the brain to be preserved and stained after death, they do not provide any information about real-time brain activity or how the brain functions while alive.
Destruction of tissue: Slicing and staining the brain is destructive, making it impossible to study the same tissue later for different purposes.
Complexity: The process of fixing, embedding, slicing, and staining brain tissue is labour-intensive and requires specialised equipment and expertise
NON-INVASIVE WAYS OF INVESTIGATING THE BRAIN
HUMAN CASES OF BRAIN DAMAGE
ADVANTAGES:
No ethical concerns: Since the damage occurs naturally or as a result of self-administered substances, there are no ethical issues related to intentionally harming the brain.
Real-world insights: Human cases provide a unique opportunity to study the real-world impact of brain damage on cognition, behaviour, and mood.
Comparison opportunities: By comparing damage effects to different brain areas across individuals, researchers can infer how specific regions contribute to overall brain function.
DISADVANTAGES:
Lack of precision: The extent and location of the brain damage are not controlled, leading to potential confounding effects.
Baseline issues: It is difficult to determine how much an individual’s behaviour has changed post-injury, as there may be no detailed records of their cognitive function or behaviour, before the brain damage.
Complexity: The brain is an integrated system, meaning damage in one region can affect the functioning of distant areas, complicating the interpretation of results.
EVALUATION OF HUMAN CASES OF BRAIN DAMAGE
ADVANTAGES:
Provides real-life, ethically sound data on how brain damage affects behaviour, cognition, and emotion.
It can offer insights that are impossible to obtain through animal studies or invasive methods in humans.
DISADVANTAGES:
Uncontrolled damage: Naturally occurring brain damage is unpredictable in its extent and location, making it difficult to isolate the role of specific brain regions.
Difficulty in baseline comparisons: Without pre-damage behavioural records, it is challenging to determine whether changes in behaviour are a direct result of brain damage.
Compensatory effects: After brain damage, other brain regions may adapt and compensate, masking the effects of the original injury.
SPATIAL RESOLUTION AND TEMPORAL RESOLUTION
In the field of brain imaging, two key factors determine the effectiveness of a scan: temporal resolution and spatial resolution.
SPATIAL AND TEMPORAL RESOLUTION: EXPLANATION AND SCAN COMPARISON
Spatial resolution refers to the ability of a brain imaging technique to distinguish between different structures or regions in terms of physical space. It determines how clearly the images can show details, helping to identify the exact location of brain activity or structural features. A higher spatial resolution means smaller brain structures can be visualised more precisely.
Temporal resolution refers to how accurately a brain imaging technique can track changes in brain activity over time. It reflects the speed with which the method can detect neural activity, which is essential for studying dynamic processes in the brain. A higher temporal resolution means quicker changes in brain activity can be captured.
BRAIN IMAGING TECHNIQUES AND THEIR RESOLUTIONS
fMRI (Functional Magnetic Resonance Imaging)
Spatial Resolution: High
Temporal Resolution: Moderate
Explanation: fMRI offers excellent spatial resolution (1-2 millimetres) for identifying active brain regions, but it has moderate temporal resolution (seconds) because it measures changes in blood flow, which are slower than neural activity.
PET (Positron Emission Tomography)
Spatial Resolution: Moderate
Temporal Resolution: Low
Explanation: PET provides moderate spatial resolution (4-5 millimetres) but has poor temporal resolution (minutes) as it tracks slower metabolic changes via radioactive tracers.
EEG (Electroencephalography)
Spatial Resolution: Low
Temporal Resolution: High
Explanation: EEG has high temporal resolution (milliseconds), making it ideal for tracking rapid neural processes, but its spatial resolution is low, making it difficult to localise the precise origin of brain activity.
ELECTROPHYSIOLOGICAL TECHNIQUES
These techniques measure the brain's electrical activity and include methods such as EEG (Electroencephalogram), ERP(Event-Related Potentials), and MEG (Magnetoencephalography). They are non-invasive and often involve placing electrodes on the scalp to record electrical signals generated by neurons.
ELECTROPHYSIOLOGICAL TECHNIQUES:
ELECTRICAL RECORDING OF THE BRAIN
OVERVIEW:
Neurons generate action-potentials and postsynaptic potentials, which can be recorded to measure the brain’s electrical activity. By recording these electrical signals, researchers can determine whether a particular brain structure or region is involved in a specific behaviour or cognitive process. There are two main methods for recording brain activity:
Microelectrodes: Small electrodes that can record electrical activity from single neurons (single-cell recording), often implanted chronically in animals.
Microelectrodes (EEG): Larger electrodes record thousands of neurons' activity simultaneously from the scalp's surface.
MICROELECTRODES (SINGLE-CELL RECORDING)
DESCRIPTION: Microelectrodes record electrical activity from individual neurons. These tiny electrodes are often implanted in an animal's brain, allowing researchers to monitor neuron activity as the animal responds to specific environmental stimuli.
ADVANTAGES:
Extremely precise: Can measure the activity of individual neurons, providing detailed information about the role of specific cells in brain function.
Real-time data: Allows researchers to observe how neurons fire in response to stimuli in real time.
DISADVANTAGES:
Invasive: Requires surgical implantation of the electrodes, making it more suitable for animal studies.
Time-consuming: Setting up the equipment and analysing single-cell data can be slow and labour-intensive.
Too focused: Measures activity in a few neurons, often neglecting the broader neuronal interactions that occur in the brain.
ELECTROENCEPHALOGRAPHY (EEG)
DESCRIPTION:
EEG measures the brain's electrical activity using electrodes placed on the scalp. It records the activity of thousands of neurons near the electrodes, and the resulting trace is known as an electroencephalogram. Due to its noninvasive nature, EEG is particularly useful for studying brain states such as sleep, wakefulness, and arousal and is frequently used in clinical and experimental settings.
RESEARCH FROM THIS TECHNIQUE: EEG has been extensively used to study changes in brain activity during different states of consciousness and in diagnosing neurological disorders like epilepsy. It also helps reveal brain activity patterns linked to cognitive processes like attention and decision-making
FOR EXAMPLE: EVALUATION:
Advantages:
Non-invasive: Electrodes are placed on the scalp, making it a safe and non-invasive technique.
High temporal resolution: EEG can detect changes in brain activity with millisecond precision, making it excellent for studying time-sensitive processes.
Differentiation between brain states: Can distinguish between different types of electrical activity, such as alpha waves (relaxed wakefulness) and beta waves (alert, focused states).
Disadvantages:
Crude spatial resolution: EEG measures the combined activity of many neurons, making it difficult to pinpoint the exact source of the brain activity.
Time-consuming: Requires significant time to set up and analyse data.
EVENT-RELATED POTENTIALS (ERPS)
DESCRIPTION:
Event-related potentials (ERPs) are specific brain responses directly related to a particular sensory, cognitive, or motor event. They are derived from EEG signals and isolated by averaging the brain’s response to a repeated stimulus over many trials. ERPs provide a more detailed view of how the brain processes stimuli and are widely used in cognitive neuroscience to study attention, perception, and decision-making.
RESEARCH FROM THIS TECHNIQUE:
ERPs have been crucial in studying how the brain processes sensory stimuli. For example, the P300 wave is an ERP component associated with attention and decision-making, while other ERP components can track visual or auditory processing.
EVALUATION:
Advantages:
Non-invasive: Like EEG, ERPs are safe and non-invasive.
High temporal resolution: Can track brain activity on a millisecond scale, revealing how quickly the brain processes stimuli.
Cost-effective: Compared to techniques like fMRI or PET, ERP research is less expensive and easier to conduct.
Disadvantages:
Poor spatial resolution: ERPs cannot pinpoint the exact brain region involved in a process due to the difficulty in tracing electrical activity back to its source.
Large sample size required: Because ERPs are small in amplitude, many trials are needed to obtain reliable results.
NON-INVASIVE BRAIN STIMULATION TECHNIQUES
These techniques stimulate brain regions to explore or influence brain function.
TRANSCRANIAL MAGNETIC STIMULATION (TMS): Uses magnetic fields to stimulate specific brain regions, often to study or treat conditions like depression and cognitive impairments.
TRANSCRANIAL DIRECT CURRENT STIMULATION (tDCS): Applies low direct current to the scalp to modulate neural activity, often used in cognitive enhancement or therapeutic interventions.
TRANSCRANIAL ALTERNATING CURRENT STIMULATION (tACS): Uses alternating current to modulate brain activity, used for research on neural oscillations and cognitive tasks.
TRANSCRANIAL RANDOM NOISE STIMULATION (tRNS): Applies random electrical noise to influence neural activity, often used in cognitive and learning enhancement research.
TRANSCRANIAL MAGNETIC STIMULATION (TMS) AND TRANSCRANIAL ELECTRICAL STIMULATION (TES)
DESCRIPTION:
Transcranial Magnetic Stimulation (TMS) uses magnetic fields to induce electrical activity in the brain. A coil is placed over the scalp, and magnetic pulses are delivered to stimulate or inhibit neural activity in specific brain regions. Transcranial Electrical Stimulation (tES), on the other hand, uses weak electrical currents to modulate brain activity. These techniques allow researchers to temporarily "lesion" parts of the brain to study their function.
RESEARCH FROM THIS TECHNIQUE:
TMS has been used to study the motor cortex, where stimulating specific areas can cause muscle contractions. It has also been used to investigate the role of the prefrontal cortex in decision-making and emotional regulation.
EVALUATION:
Advantages:
Non-invasive: TMS and tES can temporarily stimulate or inhibit brain activity without surgery.
Functional lesioning: These techniques allow researchers to temporarily deactivate brain areas to study their roles, mimicking the effects of permanent lesions but without lasting damage.
Disadvantages:
Surface-only stimulation: TMS is limited to stimulating areas on the brain's surface; deeper structures are out of reach.
Muscle cramps: Stimulation near muscles (e.g., in the scalp or face) can cause twitching, which may interfere with experiments.
Difficulty in localisation: Similar to lesion studies, it can be challenging to precisely localise which structures are affected by the stimulation.
This overview covers various electrical recording techniques for measuring brain activity and evaluating their strengths, limitations, and contributions to neuroscience. Each method, from EEG to TMS, plays a crucial role in understanding the brain’s electrical activity, providing insights into both the brain's structure and function.
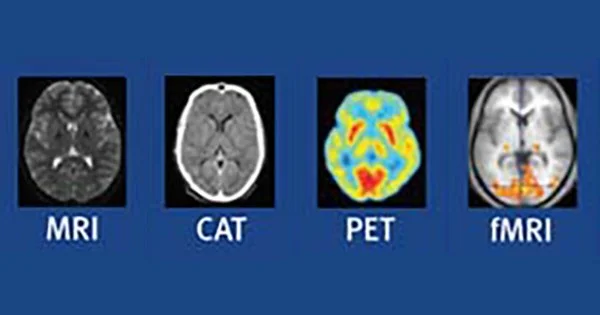
SCANS (NEURO-IMAGING)
Neuro-imaging techniques are classified into structural and functional imaging, each with its respective examples.
STRUCTURAL IMAGING TECNIQUES
COMPUTERISED AXIAL TOMOGRAPHY (CAT OR CT SCAN)
DESCRIPTION:
A CT scan builds a detailed brain image by measuring X-rays' differential absorption. The patient lies on a table that slides in and out of a cylindrical apparatus. X-rays are emitted from a source that circles the head, and the resulting signals are detected by sensors positioned around the head, creating a cross-sectional brain image.
ADVANTAGES:
Quick: Most partial CT scans take seconds, and a full-body scan can be completed in about 30 minutes.
Diagnostic versatility: The CT scan can simultaneously record images of bone, soft tissue, and blood vessels, offering clearer insights than standard X-rays.
Useful for surgery: CT scans help guide surgeons during operations and help assess the impact of therapies (e.g., radiation on tumours).
Painless: The procedure is non-invasive and painless.
DISADVANTAGES:
Radiation exposure: CT scans deliver a high dose of radiation, raising concerns about cancer risks, especially with repeated use.
Structural information only: CT scans provide static images of brain structure but do not capture brain function.
Resolution limitations: While CT offers better resolution than traditional X-rays, it is inferior to MRI in visualising finer details.
Expensive: The procedure is costly and may not be readily available in all healthcare settings.
MAGNETIC RESONANCE IMAGING (MRI)
DESCRIPTION:
MRI produces detailed images of the brain's structure by utilising a strong magnetic field and radio waves. It measures the density of hydrogen atoms in brain tissues, with the resulting differences in signal intensity allowing for the differentiation between various tissue types. MRI is commonly used to detect tumours, injuries, and abnormalities in brain structure.
ADVANTAGES:
High spatial resolution: MRI offers highly detailed brain images, making it ideal for detecting subtle abnormalities.
No radiation: Unlike CT, MRI does not use ionising radiation, reducing health risks.
Comprehensive structural comparison: MRI can compare brain structures across different groups (e.g., males vs. females, older vs. younger individuals) and in cases of abnormal vs. normal functioning.
DISADVANTAGES:
Expensive: MRI scans are costly and require specialised equipment.
Motion sensitivity: The patient must remain completely still during the scan; even small movements can distort the images.
No functional insight: MRI provides static images of the brain’s structure and cannot show brain activity.
FUNCTIONAL IMAGING
NEAR-INFRARED SPECTROSCOPY (NIRS)
DESCRIPTION:Near-infrared spectroscopy (NIRS) is a noninvasive technique that uses infrared light to measure brain activity, particularly in the surface regions of the cortex. It is also a functional brain imaging technique. It measures brain activity by monitoring changes in blood oxygen levels using near-infrared light, typically at the surface of the cortex. NIRS is used in cognitive research, especially in populations where more invasive techniques might be complex (e.g., infants). NIRS can provide information about blood oxygenation and haemodynamic responses linked to neural activity. It's less sensitive than fMRI for deep brain structures but more straightforward and inexpensive.
RESEARCH FROM THIS TECHNIQUE:
NIRS is often used in developmental neuroscience and clinical settings to study brain function in infants and patients who cannot undergo more invasive procedures. It has also been applied to investigate cognitive functions in healthy adults, such as attention and language processing.
EVALUATION:
Advantages:
Non-invasive: Like EEG, NIRS is a non-invasive and relatively easy-to-use technique.
Portable: NIRS equipment is more portable than fMRI or PET scanners, making it suitable for a wider range of settings.
Disadvantages:
Limited to surface regions: NIRS can only measure activity near the brain's surface and cannot penetrate deeply into subcortical regions.
Lower resolution: NIRS can provide information about brain activity, but it has lower spatial resolution than fMRI.
SPECT (SINGLE PHOTON EMISSION COMPUTED TOMOGRAPHY)
DESCRIPTION:
SPECT (Single Photon Emission Computed Tomography) is a minimally invasive functional brain imaging technique that uses a radioactive tracer to measure blood flow and metabolic activity in the brain. A small amount of a radioactive substance is injected into the bloodstream, which emits gamma rays detectable by a gamma camera that rotates around the patient's head. This produces 3D images that show how blood flows through various brain regions, making SPECT useful for diagnosing conditions like epilepsy, stroke, dementia, and brain injuries by identifying areas of altered brain function.
ADVANTAGES:
Functional insight: SPECT provides real-time information on brain activity, helping identify abnormalities in blood flow or metabolism.
Detects abnormalities missed by structural imaging: Conditions like epilepsy or dementia, which may not be visible on structural scans (e.g., MRI or CT), can be detected with SPECT due to changes in brain function.
Widely available: SPECT is less expensive and more widely accessible than PET scans, making it more common in clinical use.
Longer tracer half-life: The radioactive tracers used in SPECT have a longer half-life, making it easier to administer than PET, which requires faster use.
DISADVANTAGES:
Minimally invasive: Since SPECT involves injecting a radioactive tracer into the bloodstream, it introduces a small amount of radiation into the body.
Lower spatial resolution: SPECT provides lower spatial resolution compared to MRI or PET, which can make it less precise in identifying small abnormalities.
Time-consuming: The process of waiting for the tracer to circulate through the body can make the scan take longer than other imaging methods.
Limited structural detail: SPECT gives functional information but does not provide the detailed anatomical data that MRI or CT scans can.
IS IT USED IN RESEARCH?:
Yes, SPECT is widely used in research to explore how blood flow and metabolism relate to brain function in different conditions:
Neurological research: SPECT is used to study blood flow in conditions like Alzheimer’s disease, epilepsy, Parkinson’s disease, and stroke, providing insights into how these disorders affect brain function.
Psychiatric research: SPECT is applied to study psychiatric disorders, such as schizophrenia, depression, and bipolar disorder, helping researchers observe abnormalities in brain function related to mood and behavior.
Drug development: SPECT is used to monitor the effects of new drugs on brain function, observing how medications impact blood flow or metabolic activity in specific brain regions.
Cognitive neuroscience: Researchers use SPECT to study how blood flow changes during cognitive tasks, such as memory, decision-making, and emotional processing, to map out the brain regions involved in these activities.
KEY DIFFERENCES BETWEEN SPECT AND PET SCANS
SPECT provides less detailed images but is more accessible and cost-effective.
PET provides more precise, high-resolution images but is more expensive and less readily available.
POSITRON EMISSION TOMOGRAPHY (PET SCAN)
DESCRIPTION: PET scans use trace amounts of radioactive material to map functional processes in the brain. A radioactive substance (tracer) is injected into the bloodstream, and the brain’s metabolic activity is visualised as the tracer decays, emitting positrons. Areas of high radioactivity indicate regions of high brain activity.
ADVANTAGES:
Functional insight: PET can detect changes in body function caused by disease before anatomical changes become evident, allowing for early diagnosis.
Tolerates slight movement: PET scans are less affected by small movements than MRI or fMRI, making it more suitable for patients who struggle to remain still.
DISADVANTAGES:
Radiation exposure: PET involves the injection of radioactive material, which poses a slight risk, especially in pregnant or breastfeeding individuals.
Time-consuming: The tracer takes 30-60 minutes to reach the target area, and the scanning process can take 45-60 minutes.
Expensive: PET imaging is costly and may not be widely available.
Limited scans: The radioactive components used in PET limit the number of times a patient can safely undergo the procedure.
Low spatial resolution: PET has relatively low spatial resolution, making it difficult to detect very small abnormalities, and it often needs to be combined with CT or fMRI for better clarity.
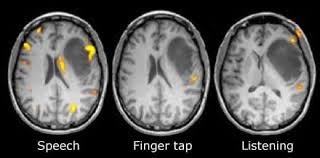
FUNCTIONAL MAGNETIC RESONANCE IMAGING (fMRI)
DESCRIPTION:fMRI measures brain activity by detecting changes in blood flow. It is based on the principle that oxygen-rich and oxygen-poor blood behave differently in a magnetic field. Active brain areas receive more oxygenated blood, and fMRI captures this change, allowing researchers to pinpoint areas of increased neural activity.
ADVANTAGES:
Non-invasive: fMRI does not involve radiation and is generally safe for repeated use.
High spatial resolution: fMRI provides detailed images of the brain, allowing for precise localisation of activity.
Objective: Compared to traditional psychological evaluations, fMRI objectively measures brain function.
DISADVANTAGES:
Expensive: The cost of fMRI is high, and it requires specialised equipment.
Motion sensitivity: The patient must remain completely still during the scan to avoid image distortion.
Poor temporal resolution: fMRI images are typically collected every 2 seconds, making them less suitable for studying fast neural processes.
Limited understanding: While fMRI reveals areas of brain activity, researchers are still learning how to interpret the data regarding specific cognitive functions.
MAGNETOENCEPHALOGRAPHY (MEG)
MEG as an Electrophysiological Technique:
MEG detects the magnetic fields generated by electrical currents in the brain, specifically from neuronal activity. Since it directly measures brain activity in real-time, it falls under the category of electrophysiology.
It is non-invasive, placing MEG in the same category as EEG, but it provides higher spatial resolution than EEG, allowing for more precise localization of brain activity.
MEG as a Functional Imaging Technique:
MEG provides functional information by mapping brain activity during various tasks (e.g., sensory processing, movement, language).
It is used to create functional brain maps, which can be particularly useful in pre-surgical planning for epilepsy or tumor resection, as well as in cognitive neuroscience research.
DESCRIPTION:
MEG measures the magnetic fields produced by electrical activity in the brain. The technique is contact-free, and magnetic fields penetrate brain tissues without distortion. MEG is used to precisely localise cortical activity sources and is often employed in both research and clinical settings.
ADVANTAGES:
Non-invasive: MEG provides a contact-free method for measuring brain activity, making it safe and non-invasive.
High temporal resolution: MEG offers high temporal precision, capturing real-time neural activity.
No tissue distortion: Magnetic fields are not distorted as they pass through the scalp, allowing for accurate localisation of brain activity.
DISADVANTAGES:
Expensive: MEG requires advanced, costly equipment and specialised facilities.
Limited spatial resolution: Although MEG offers excellent temporal resolution, it has limited spatial resolution, making it difficult to localise activity in deeper brain structures.
EVALUATION OF SCAN METHODS
STRUCTURAL IMAGING (CT, MRI):
Advantages: Provides detailed images of the brain's anatomy, aiding in diagnosing structural abnormalities (e.g., tumours, injuries).
Disadvantages: It cannot show brain function or activity, and some techniques (CT) involve radiation exposure.
FUNCTIONAL IMAGING (PET, fMRI, MEG):
Advantages: Offers real-time insight into brain activity, helping researchers understand how different brain regions function during specific tasks.
Disadvantages: Functional imaging techniques can be costly, and some (e.g., PET) involve using radioactive tracers.
While most medical scans like MRI and CT are considered safe, some functional imaging techniques like PET (Positron Emission Tomography) and SPECT (Single Photon Emission Computed Tomography) do carry certain risks due to the use of radioactive tracers. Repeated exposure to ionizing radiation can increase cancer risk, which is why some countries limit how frequently patients can undergo these procedures. For example, the International Commission on Radiological Protection and the European Commission recommend limiting PET and SPECT scans when feasible, especially in non-urgent cases
LASTLY
Other methods like gene sequencing or computer science techniques aren’t typically included because they study genetics, DNA, or computational models rather than direct brain activity or structure. Gene sequencing is more relevant to genomics and understanding predispositions to brain disorders. At the same time, computer science contributes to developing tools (e.g., neural networks) but doesn’t directly measure or image the brain. Things like Carbon dating are used to date ancient biological materials unrelated to brain function studies.
ASSESSMENT
Q1: Which of the following is not a way of studying the brain?
Circle one answer only (Total: 1 mark)
A Electrocardiogram (ECG)
B Electroencephalogram (EEG)
C Event-related potentials (ERPs)
D Functional magnetic resonance imaging (fMRI)
Q2: Which method of studying the brain would most accurately identify specific brain areas activated during a cognitive task?
Circle one answer only (Total: 1 mark)
A Electroencephalogram (EEG)
B Event-related potentials (ERPs)
C Functional magnetic resonance imaging (fMRI)
D Post-mortem examinations
Q3: Which of the following is a feature of functional magnetic resonance imaging (fMRI)?
Circle one answer only(Total: 1 mark)
A Directly measuring the electrical activity of neurons using electrodes implanted in the brain.
B Directly measuring the electrical activity of neurons using electrodes on the scalp.
C Indirectly measuring the electrical activity of neurons by recording changes in brain blood flow.
D Indirectly measuring the electrical activity of neurons by recording changes in neurotransmitter release.
Q4: The electroencephalogram (EEG) and event-related potentials (ERPs) both involve recording the electrical activity of the brain. Outline one difference between EEGs and ERPs. (Total: 2 marks)
Q5: Explain one difference and one similarity between functional magnetic resonance imaging (fMRI) and event-related potentials (ERPs) as ways of studying the brain. (Total: 4 marks)
Q6: Briefly evaluate the use of EEGs as a way of identifying cortical specialisation in the brain. (Total: 3 marks)
Q7: Outline and evaluate one or more ways of studying the brain. (Total: 8 marks)
Q8: Discuss ways of studying the brain. (Total: 16 marks)